ЫмСЯПЩвдЫЕЪЧДѓздШЛЬьЩњЕФГ№ШЫ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌПЩЪЧШЫРрЩчЛсгжВЛПЩЭбРыЫмСЯ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЖрИіВњЦЗжаЖМашвЊЪЙгУДѓзкЕФЫмСЯЁЃ�ЁЃХЗУЫROHSЮЊСЫядЬЫмСЯЙигкШЫЬхЕФЮЃЯевдМАЖдДѓздШЛЕФЮлШО�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌзЈУХНЈЩшСЫбаОПЪЕбщЪв�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЭЈЙ§жиИДЕФЪЕбщзюжеЕУГіЫмСЯЕФЧхОВЯожЕЁЃ�ЁЃВЂАбЯожЕзіЮЊЫмСЯROHSВтЪджИСюЁЃ�ЁЃдкББУРКЭХЗжоаэЖрЙњМвАбROHSФЩШыВњЦЗЧхОВЗЈжа�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌВњЦЗЮДЭЈЙ§ROHSВтЪдВЛдЪаэдкЪаГЁЩЯЯњЪл�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЯТУцЮвУЧРДНщЩмЫмСЯROHSВтЪдЁЃ�ЁЃ

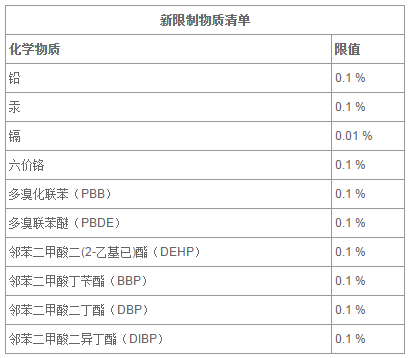
ROHSЫмСЯЦЪЮідЊЫиЃКCr, Mn, Fe, Ni, Cu, Zn, Hg, As, Pb, Se, Rb, Sr, Zr,CoЁЂVЁЂ Mo, Ag, Cd, Sn, Sb, Ba ,K, Ca, Ti�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌThЕШМИЪЎжждЊЫиЃЛ�ЃЛ�ЃЛ
ЫмСЯROHSПЩВтЖЈАќРЈЧсдЊЫи,ЪЪгУгкЖржжН№ЪєЛљЬх�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌШчЃКЬњЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌТСЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЭЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌФјЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌИѕЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌюбЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌУОЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌаПЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЮ§ЛљКЭЧІЛљ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌетаЉН№ЪєЛљЬхЖМЪЧЖдШЫЬхКЭздШЛЧщаЮгаНЯДѓЦЦЫ№ЕФгаКІЮяжЪЁЃ�ЁЃ
ROHSаТЯожЦЮяжЪЧхЕЅЃК
ХЗУЫROHSгЩдРДЕФЁАROHSСљЯюЁБИќаТЮЊЯждкЕФЁАROHSЪЎЯюЁБ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌROHSВтЪдБЛГЦЮЊШЋЧђТЬЩЋЛЗБЃжИСю�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌдкКмДѓЕФЫЎЦНЩЯАќЙмСЫВњЦЗЕФЧхОВЁЃ�ЁЃХЗУЫROHSЫфШЛЫЕВЛЪЧЧПжЦадВњЦЗЧхОВШЯжЄ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌПЩЪЧВњЦЗашвЊНјШыХЗУЫЁЂББУРЁЂФЯЗЧЕШЕиЧјЖМашвЊзіROHSВтЪдЁЃ�ЁЃЖрИіЕиЧјАбROHSВтЪдФЩЮЊЧПжЦадВњЦЗЧхОВШЯжЄЗЈ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌВњЦЗНјШыИУЕиЧјБиашЭЈЙ§ROHSВтЪдЁЃ�ЁЃ
дкЭтбѓаэЖрДѓЦѓвЕдкВЩЙКЩЬЦЗЪБЖМашвЊЦѓвЕАьРэROHSВтЪд�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌШєЪЧФњЕФВњЦЗашвЊдЖЯњЭтбѓФЧУДROHSВтЪдвЛЖЈЪЧВЛПЩвдзшжЙЁЃ�ЁЃЭђЭђВЛвЊБШМАЭтбѓПЭЛЇРДСЫВХЯыЕНашвЊЮЊВњЦЗзіROHSВтЪд�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌФЧбљжЛЛсЮЊЦѓвЕДјРДИќЖрЕФАУФеЁЃ�ЁЃ
ЫЕЕРСЫетРяБиВЛПЩЩйЕФашвЊНщЩмROHSВтЪдгУЖШ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЙигкгУЖШетИіЮЪЬтЪМжедкЫЕШДЪМжеЮоЗЈИјЖСепвЛКаПЯЖЈЕФУеЕз�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌВЛЪЧаЁБрФдзгЧЗКУЪЙ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЖјЪЧетИіЮЪЬтЪМжеЫЕВЛЧхЮњ�ЃЌ�ЃЌ�ЃЌ�ЃЌ�ЃЌЃЌЮЊВњЦЗзіЙ§МьВтШЯжЄЕФЦѓвЕПЯЖЈОЭУїШЗЁЃ�ЁЃдкУЛгаПДЕНВњЦЗЯъЯИаХЯЂЪБЫвВЮоЗЈИјВњЦЗвЛИізМШЗЕФгУЖШЁЃ�ЁЃОЭЫуИјГідЄЙРМлЧЎФЧУДгыЯжЪЕМлЧЎПЯЖЈвВЪЧЯрВюНЯдЖЁЃ�ЁЃШчашЯрЪЖВњЦЗАьРэROHSВтЪдЯъЯИашвЊМИЖргУЖШЧыЕуЛїгвВрдкЯпПЭЗўОйаазЩбЏЁЃ�ЁЃ










 ЮЂаХзЩбЏ
ЮЂаХзЩбЏ аХЯЂЬсНЛРжГЩ
аХЯЂЬсНЛРжГЩ